Your silicone component failed after high-temperature exposure1, and you need to know why. Was it melting, burning, or something else entirely? Understanding the true failure mode is critical for your design.
Silicone, a thermoset material, does not melt like plastic. At high temperatures, it undergoes degradation, a chemical breakdown that causes it to lose its mechanical properties and become brittle. At extreme temperatures, it can burn, but this process is more about meeting specific flame-retardant certifications.

Many designers I talk to think about high-temperature failure in simple terms, like melting. But the reality for Liquid Silicone Rubber (LSR)2 is much more complex and, frankly, more dangerous for your product's reliability. The true failure modes are often invisible until it's too late. To design robust parts, you need to look past the idea of melting and understand what really happens when your silicone component gets hot. Let's break down the real killers: degradation and burning.
Does silicone degrade at high temperature?
You see your silicone parts failing after exposure to heat. You're not sure if they are just getting old, or if the heat is actively destroying them. The truth is, heat is the primary trigger.
Yes, high temperatures absolutely cause silicone to degrade. This is not melting; it is a chemical breakdown. The material loses its strength and elasticity, becoming brittle. This sudden "performance cliff" is the real danger for designers, often leading to unexpected product failures in the field.
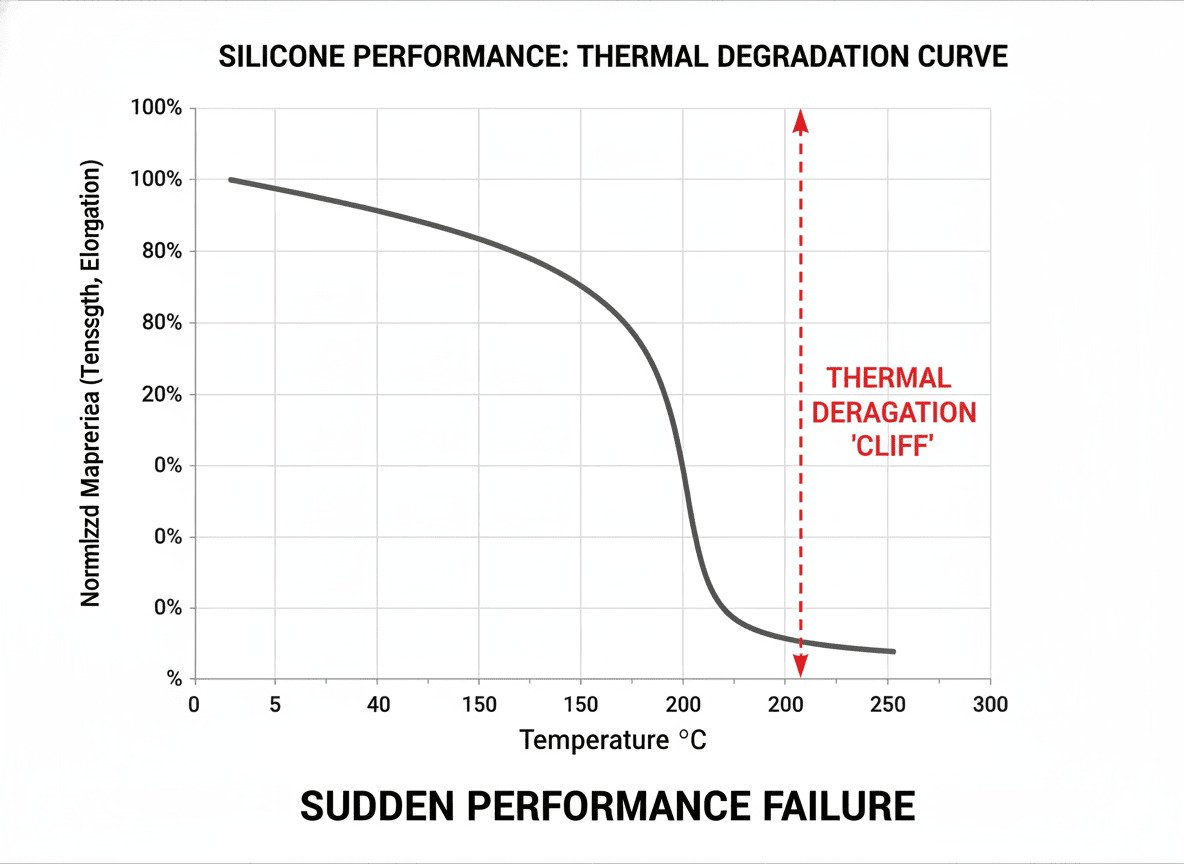
I always tell my clients that LSR will never "melt." As a thermoset material, its polymer chains are cross-linked together in a fixed, three-dimensional structure. You can't just heat it up and expect it to turn into a liquid like a thermoplastic. Instead, when you push it beyond its thermal limits, you trigger a process called degradation. This is where the material's performance falls off a cliff. It might look fine on the outside, but internally, it's suffering from a kind of "internal injury" that leads to catastrophic failure.
I remember a case with a European client who makes high-end kitchenware. They designed a new pressure cooker lid with an LSR seal. The design required the seal to perform reliably in a 150°C steam environment for years. The initial samples they received passed all their tests. But a few months into mass production, customer complaints about leaking lids started pouring in. We investigated and traced the problem back to the manufacturing process. The original supplier, trying to save money, had cut the post-curing process from the required four hours down to just one. This shortcut meant the cross-linking process was incomplete, and volatile, low-molecular-weight siloxanes were left inside the material. In the hot, steamy environment of the pressure cooker, these residuals accelerated the material's degradation, causing its compression set properties to fail. The seal could no longer bounce back, and it started to leak. This highlights how a hidden process failure can lead to a very public product failure.
Thermoset vs. Thermoplastic Behavior
| Property | Thermoset (e.g., LSR) | Thermoplastic (e.g., Polypropylene) |
|---|---|---|
| Structure | Cross-linked polymer chains | Non-cross-linked polymer chains |
| Behavior Under Heat | Does not melt; degrades and becomes brittle | Melts and can be re-molded |
| Recyclability | Cannot be re-melted or recycled easily | Can be melted and recycled |
| High-Temp Failure | Loss of mechanical properties (degradation) | Softening and melting |
What causes silicone to degrade?
Your silicone parts are failing under heat, but you need to know the exact mechanism. Is it just the heat, or are other factors making things worse? Degradation is a chemical process with specific triggers.
Silicone degradation is mainly caused by a combination of high heat, oxygen, and time. This process, known as thermo-oxidative degradation, breaks down the polymer’s strong siloxane (Si-O) backbone. Impurities, catalysts, or constant mechanical stress can significantly accelerate this breakdown, leading to premature failure.
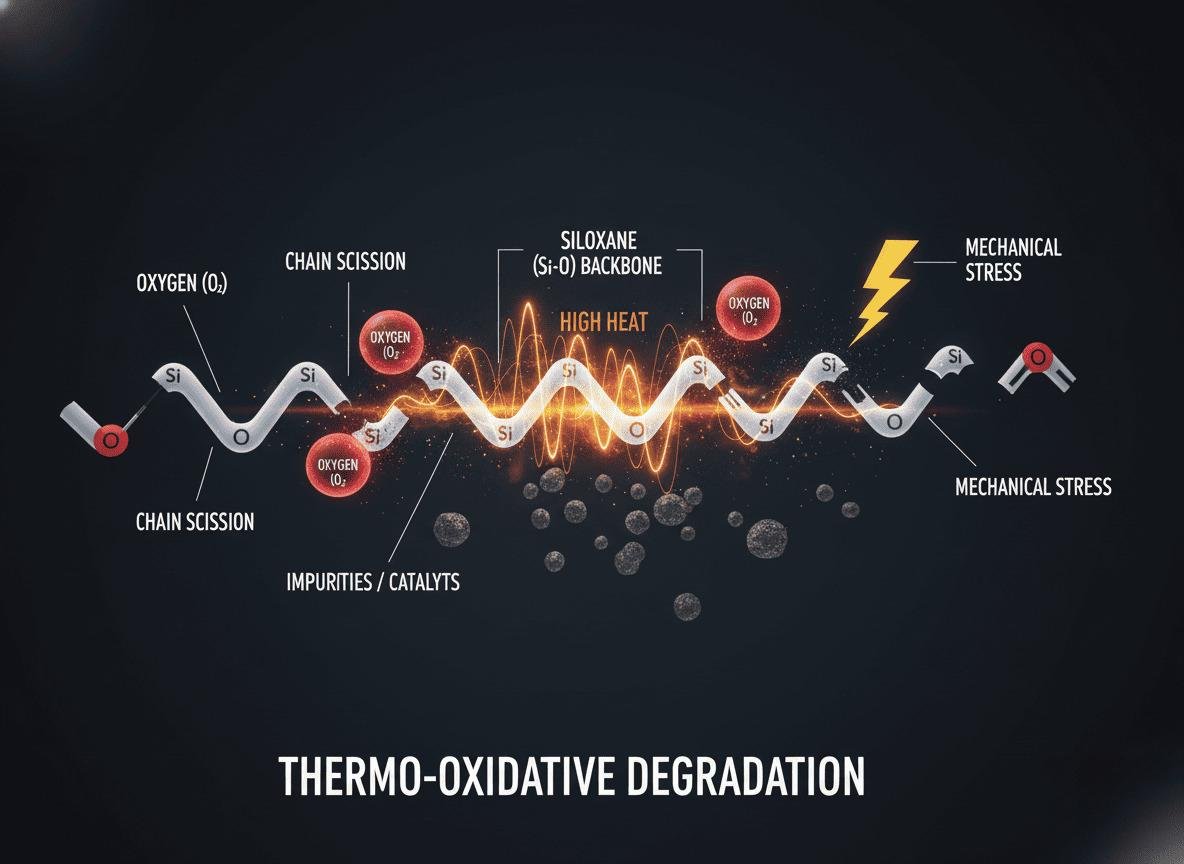
The incredible thermal stability of silicone comes from its backbone, which is made of strong silicon-oxygen (Si-O-Si) bonds. These bonds are much stronger than the carbon-carbon bonds found in most organic plastics. However, they are not invincible. At high enough temperatures, typically above 200-250°C for standard grades, these bonds can be attacked, especially when oxygen is present. This is the essence of thermo-oxidative degradation. The process starts when heat creates reactive free radicals on the polymer chain. These radicals then react with oxygen, creating a chain reaction that attacks other parts of the polymer. This reaction can either break the main chain, making the material softer, or create more cross-links, making it harder and more brittle. For most LSR applications I’ve seen, embrittlement is the far more common and dangerous outcome.
Key Factors Accelerating Degradation
Several factors can make this degradation happen faster and at lower temperatures. As a designer, you need to be aware of them.
- Catalyst Residues: In LSR injection molding, we use a platinum catalyst to cure the material. If the mixing ratio is off or the process isn't controlled, residual catalyst can remain in the part. These residues can promote degradation later in the product's life.
- Impurities: The purity of the raw silicone material is critical. Certain metals, like iron or copper, or even acidic or basic compounds trapped in the material, can act as catalysts, speeding up the breakdown of the polymer chain.
- Mechanical Stress: A part that is under constant stress, like a compressed gasket or a stretched tube, will degrade much faster at high temperatures than a part that is at rest. This phenomenon is called stress relaxation and is a major failure mode for seals.
- Operating Environment: Heat alone is one thing. But heat combined with steam, aggressive chemicals, or UV radiation can create a synergistic effect that attacks the material from multiple angles, causing it to fail much sooner than expected.
Will high temperatures burn silicone?
You are designing a part for an electronic device and are worried about it catching fire. You see smoke or charring in tests and assume it's burning like plastic. The reality is more about smoldering and meeting standards.
Yes, silicone will burn at very high temperatures, but its behavior is completely different from plastics. It tends to smolder and forms a non-conductive silica ash instead of melting and dripping flaming liquid. For designers, the real question is not if it burns, but if it meets specific flame-retardant certifications3.

In my experience working with clients in the electronics and automotive industries, the "burning" of silicone is not a technical question but a market access question. It’s about certification. If your product needs to be sold in Europe or the US, its components must meet specific safety standards for flammability. This is a simple pass/fail issue. If your silicone part doesn't have the right certification, your product cannot legally be sold, period.
Unlike plastics that melt, drip, and spread flames, silicone behaves very predictably. When exposed to a flame, it doesn't melt. Instead, it slowly burns and decomposes into silicon dioxide (SiO2), which is essentially a fine, white ash. This ash is important because it forms a crust that acts as a thermal barrier, slowing down further burning. It's also electrically insulating, which is a huge advantage in electrical fires. The smoke produced is generally less toxic than that from burning hydrocarbons.
Why Certification is the Real Issue
For a product designer like Michael, the most important standard to know is UL 944. This is the test that determines the flammability rating of plastic and rubber materials. You don't choose a silicone based on whether it burns; you choose a grade that is certified to the UL 94 rating your application requires.
| UL 94 Rating | Description | Common Application |
|---|---|---|
| HB (Horizontal Burn) | The material burns slowly on a horizontal specimen and self-extinguishes. | General purpose parts, not for enclosures. |
| V-1 (Vertical Burn) | Burning stops within 30 seconds on a vertical specimen. Drips are allowed, but they must not be flaming. | Connectors, some consumer electronic casings. |
| V-0 (Vertical Burn) | Burning stops within 10 seconds on a vertical specimen. Drips are allowed, but they must not be flaming. | Critical applications like EV battery seals, power supply enclosures, and medical devices. |
Choosing a V-0 certified LSR grade is not a suggestion; it's a mandatory requirement for many high-performance applications. It is a true "0 or 1" problem for getting your product to market.
What happens to silicon when exposed to high temperatures?
You see physical changes in your silicone part after it has been heated. You are not sure what these changes mean for the part’s performance or reliability. Each visual change indicates a specific stage of breakdown.
When exposed to high temperatures, silicone undergoes a sequence of physical changes. Initially, it may become harder and less flexible. With prolonged exposure, it becomes very brittle and develops cracks. At extreme temperatures, it will char and finally turn into a white or grey silica ash.

By observing a silicone part, you can often diagnose the extent of its thermal damage. The changes it goes through follow a predictable timeline from reversible effects to complete destruction. Understanding this timeline helps you determine if a part has been pushed past its limits.
The Visual Timeline of Thermal Failure
Stage 1: Reversible Changes (Within the Operating Range) At temperatures within the material's continuous use rating (e.g., below 200°C for a standard grade), you might see slight softening or swelling. These effects are often temporary. The polymer chains have more energy and move around more freely, but no permanent damage occurs. Once the part cools down, it should return to its normal state.
Stage 2: Irreversible Degradation (Beyond the Operating Range) Once you exceed the temperature limit, even for a short time, permanent damage begins.
- Hardening and Embrittlement: This is the most common failure mode you'll see. The material loses its flexibility and feels stiff. If you try to bend it, it will crack or snap instead of flexing. This is caused by oxidative reactions that create additional cross-links in the polymer.
- Loss of Compression Set: For seals, this is the silent killer. The part loses its ability to bounce back to its original shape after being compressed. This leads to leaks.
- Color Change: The part may start to yellow or darken as the polymer structure begins to break down.
Stage 3: Decomposition (At Extreme Temperatures) At very high temperatures (e.g., above 350-400°C), the material starts to fall apart completely.
- Charring: The part turns black as the organic methyl groups (CH3) on the polymer backbone burn away.
- Ash Formation: After the organic components are gone, all that remains is the silicon-oxygen structure, which converts to a brittle, white or grey silicon dioxide (SiO2) ash.
| Temperature Range | Observed Effect | Underlying Cause | Reversible? |
|---|---|---|---|
| Continuous Use (e.g., < 200°C) | Minimal change, slight softening | Temporary polymer chain movement | Yes |
| Intermittent Peak (e.g., 200-300°C) | Hardening, embrittlement, cracking | Irreversible thermo-oxidative degradation | No |
| Extreme (e.g., > 350°C) | Charring, conversion to ash | Complete decomposition of the polymer | No |
Conclusion
In summary, silicone does not melt. It fails through degradation, a chemical process that makes it brittle and ruins its mechanical properties. Burning is a separate issue, governed by certifications like UL 94. Understanding these distinct failure modes is essential for designing reliable, safe, and long-lasting silicone components.
Explore the consequences of high-temperature exposure on silicone and how to mitigate risks. ↩
Discover the advantages of LSR in creating durable and flexible components. ↩
Discover the importance of flame-retardant certifications for safety in product design. ↩
Understand the UL 94 standard and its relevance for silicone materials in various applications. ↩


